Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A Brief Survey on the Advances of COVID-19 Treatment: With a New Suggestion to Capture Coronavirus by Magnetic Nanoparticles
*Corresponding author: Reza Taheri Ledari and Ali Maleki, Department of Chemistry, Catalysts and Organic Synthesis Research Laboratory, Iran University of Science and Technology, Tehran, Iran.
Received:June 07, 2022; Published:July 06, 2022
DOI: 10.34297/AJBSR.2022.16.002270
Abstract
In December 2019, a new disease emerged in China (Wuhan) and quickly spread to other countries. It is called “COVID-19” and soon became the world’s first concern due to the large number of daily victims (COVID-19 stands for coronavirus disease 2019). During this time and until the Definitive vaccine for COVID-19 be produced, researchers have to find the best therapeutic method to rescue the patients from death. In this regard, so many efforts have been made and several treatment/prophylaxis methods have been suggested, during this time (one year). In this survey, we try to collect and submit a brief report of the significant advances in treatment of COVID-19. Along that, a convenient treatment method by using of the iron oxide magnetic nanoparticles and the fake angiotensin-converting enzyme 2 (ACE2) (the receptor of the coronavirus) is theoretically suggested, which seems to be applicable.
Keywords: Angiotensin-converting enzyme 2; Omicron; Nano-biotechnology; SARS-CoV-2; Vaccines; Mutations.
Introduction
COVID-19 disease (COVID-19 stands for coronavirus disease 2019), is caused by the acute respiratory syndrome of SARS-CoV-2 virus. This disease appeared in December 2019 in China (Wuhan) and spread first throughout China and then in all over the world. On January 31, 2020, the World Health Organization (WHO) declared the epidemic a public health emergency [1]. Symptoms of this disease are fever, cough, fatigue, shortness of breath, pneumonia in the moderate and severe stage, and its Appears by systemic organ involvement including, among others, the heart, kidneys, liver, intestines, the muscular and nervous system in the critical stage, and in some cases leads to death [1,2]. In mid-January 2020, when the Chinese New Year celebrations began, a number of people living in Wuhan left their hometown for the holidays, and the virus spread to cities and other countries [3]. Upon disease spread, researchers immediately started working on it and found out that the coronavirus is from a similar species with severe acute respiratory syndrome (SARS), which was a viral disease with high transmission and pathogenicity, and was spread in China (Quang Dong), for the first time in 2002 [4]. A decade after the outbreak of SARS coronavirus (SARS-COV), a new pathogenic virus called Middle East respiratory syndrome (MERS) was first identified in Saudi Arabia (in 2012), and then spread in the Middle East’s countries [5]. According to the report announced by the WHO, from December/2019 till now (January 2022) 312,173,462 people were infected with COVID-19 and 5,501,000 people lost their lives, which is terrible statistics. According to this account, the American countries are known as the most affected areas and the Africa continent is known as the least affected area [6]. Worst of all, around 25.9% of the infected people to COVID-19 need intensive care unit (ICU) hospitalization, but unfortunately there are not enough facilities in the countries and their lives are in danger [7]. Research have revealed that men are more susceptible to this disease than women, and people over the age of 60 are more at risk of die [7,8].
From the structural aspect, coronavirus is a crown-like polymer with positive-sense and single-stranded RNA with the mean size 120nm [6,9]. The key-like paradigm as the same as antibody-antigen is passed by angiotensin-converting enzyme -2 (ACE2), through which the coronavirus is hooked into and entered in the lung’s cells [10]. The onset of the disease causes respiratory failure due to alveolar injury [10,11]. Generally, there are two subunits in the coronavirus’s structure through which the virus could be attached to the cell membrane and internalized. Briefly, subunit I (receptorbinding domain) interacts with the N-terminal peptidase domain (Ser19-Asp615) at ACE2 and is dissociated. It causes subunit 2 to rapidly cross from a metastable pre-fusion to a more-stable postfusion state that is essential for membrane fusion [12,13]. Hence, it can be expressed that attachment to the ACE2 is a crucial stage for coronavirus to enter into the lung’s cells. Accordingly, some treatments can be proposed in which ACE2 is manipulated and subjected to the infected blood.
Herein, we intend to make a brief review on the significant progresses in COVID-19 therapy achieved by the researchers, during these one year since the onset of the disease. Moreover, since we are experts in the field of magnetic nanoparticles, an applicable treatment method came to our mind that seems to give desired result, if be tried practically.
Main Discussion
Origin and Epidemiology of SARS-CoV-2
The outbreak of coronavirus began at the seafood market, where foods such as bats, frogs, snakes, birds and rabbits were sold [14,15]. Research have disclosed that most people with the disease have nothing to do with the seafood market. After a while, the researchers found out that the disease is transmitted through people communications and people being close to each other through nasal and mouth breathing [14-16]. An epidemiological study reported that eight out of nine patients were associated with the seafood market in Wuhan [16]. Some of the people did not go to the seafood market but they were staying at a nearby hotel. These findings gave the thread that transmission of the disease through droplets is possible [17]. Infection of the family and medical staff has also confirmed the transmission of the disease from human to human [14-17]. Bats have been cited as the primary source of coronavirus because genetic analysis has revealed that SARS-CoV is genetically related to acute respiratory syndrome in bats [5,14,15]. Phylogenetic analysis has shown that coronaviruses were derived from bats [18,19]. But there is evidence that another animal was a host between humans and bats [4, 20]. As the first witness it can be referred that the bats were hibernating in late December of 2019, and there were no bats in the Wuhan (China) seafood markets [16]. According to a recent experimental report (performed by Zhang et. al), pangolin-COV genome showed 91.02% nucleotide similarity with the SARS-CoV-2 genome [4,16,20]. It was found out from a practical investigation on dead Malayan pangolins.
Typology of SARS-CoV-2
Coronaviruses are very small in size and also have a singlestranded RNA as a nucleotide material. These viruses cause lung involvement as well as death [21]. In fact, the coronavirus mainly causes respiratory infections and problems in the digestive system. This is genetically divided into four categories namely alpha, beta, gamma, and delta. The first two types infect mammals, and the second two types infect the birds and mammals [22,23]. So far, two types of the alpha-coronavirus including H-CoV-Nl63 and H-CoV-229E, and four types of the beta-coronavirus including complete H-CoVOC43 and H-CoV-KU1, SARS-CoV, MERS-CO, and SARS-CoV-2 have been identified [23]. From topology perspective, the coronavirus is a spherical complex of the various protein species including spike (onto the surface), membrane (onto the surface), envelop (in the membrane), nucleocapsid (inside the sphere), and singlestranded proteins (in the center) (Figure 1) [6]. Spike glycoprotein located on the outer surface of the coronavirus and is responsible for the specific attachment of the coronavirus to the ACE2 receptor through its crown-like structure [24]. This component is divided into two main subunits, S1 and S2. The S1 subunit consists of three regions (A, B and C) that bind to the host cell receptors [6,24,25]. For example, CoV-OC43 and CoV-KU1 enter the target cell from region A of subunit S1 and MERS-CoV from both regions A and B, SARS-CoV and SARS-CoV-2 through direct interaction with domain B [7]. The beta-coronavirus genome encodes several structural proteins, such as glycosylated spike protein (S), which functions as a major inducer of host immune responses. This S protein mediates the entry of SARS-CoV-2 into the host cell by binding to a receptor protein called the angiotensin-converting enzyme (ACE2) in the surface membrane of host cells. It has been reported that, this entry process requires S priming protein, which is facilitated by the serine protease which produced by the TMPRSS211 host. In addition, RNA-dependent RNA polymerase (RdRp), the major coronavirus protease (CLpro 3), and papain-like protease (PLpro) are nonstructural proteins that the viral genome also encodes them. As soon as entering the host cells, the viral genome is released as a single-stranded positive RNA. Then, it is translated into several viral proteins via using a host cell protein translation machine, which are then broken down into effective proteins by the viral proteins 3CLpro and PLpro. PLpro also behaves as a deubiquitinase, which may deubiquinate some host cell proteins, including interferon factor 3 and NF-κB which leading to immunosuppression. RdRp synthesizes a full-length negative strand RNA template to be used by RdRp to make more viral genomic RNA. The interaction between viral S protein and ACE2 on the surface of the host cell has been of great interest since the onset of the infection process [26]. As shown in Figure 2, after internalization of the coronavirus into the lung’s cell via endocytosis process, the virus releases its RNA [7,27]. The structure of the spike protein in SARS-CoV (spread in 2002) and SARS-CoV-2 (spread in 2019) slightly differ from each other. In fact, some mutations in SARS-CoV receptor binding sites resulted in the formation of the SARS-CoV-2 [6]. In SARS-CoV-2, receptor binding domain (RBD) of S1 subunit binds to the angiotensin receptors located on alveolar cells, and the S2 subunit facilitates the combination between the host cell membrane and the virus [25,27]. The alveolar cells located on the surface of the lung are divided to three main sections: the first one is responsible for the gas exchange; the second part is composed from protein and lipid and is responsible for the production of surfactant. The angiotensin receptors are present in this part of alveolar cells. The third part contains macrophages (immune cells) [7]. When the coronavirus binds to the angiotensin receptors on alveolar cells, macrophages secrete cytokines by stimulating immune cells [6,7]. A very important question is this: why patients with an underlying disease of diabetes are at higher risk of death by COVID-19? The experimental results have shown that some enzymes such as LDH, HBDH, ALT, GGT in the blood serum of the infected people exceed from the normal range and as a result the internal organs such as myocardium, kidney and liver are damaged [28]. That is why some patients have died from multiple organ injuries [28]. The coronavirus exacerbates diabetes by over-regulating glucose metabolism, and this affects the severity of pneumonia [29] (Figure 2).
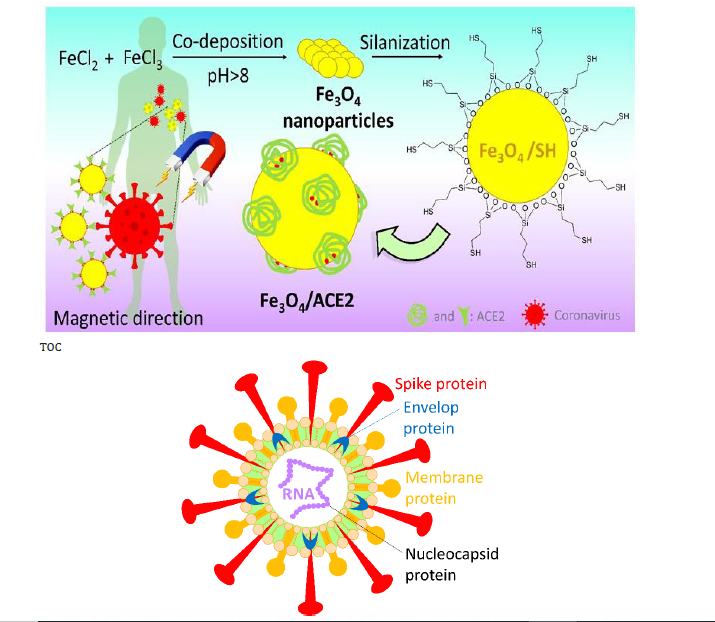
Figure 1: General morphology of a coronavirus (some proteins on the surface of coronavirus and single-stranded RNA in the center of virus).
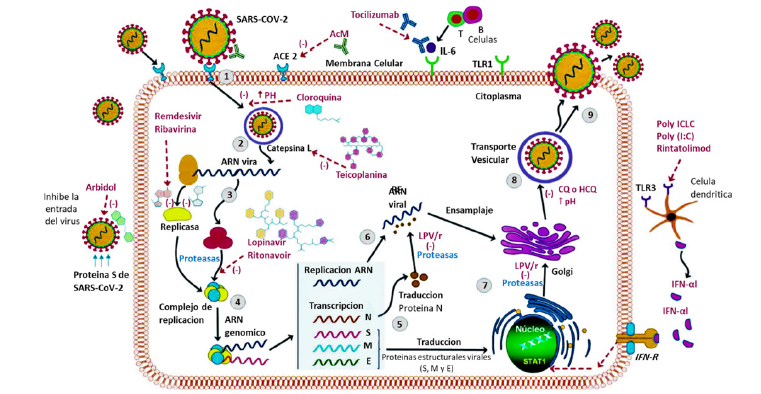
Figure 2: Virus Life Cycle: ACE2 link allows the virion to enter the cytoplasm (endocytosis) and then release its RNA, which is also introduced directly by the cell membrane during the fusion process [30].
Diagnosis of SARS-CoV-2
Almost in all the patients, hypogeusia (decrease in smell)
and hyposmia (decrease in taste) have been reported as the
early symptoms of COVID-19 [31]. One of the topics that many
researchers are considering today due to the diversity and
prevalence of coronavirus is the design of methods for accurate and
rapid diagnosis of this virus to control and treat it quickly. To date,
the tests available to detect Covid 19 can be divided into two main
categories:
a. Molecular testing is a method based on the identification of
viral RNA
b. Serological tests that measure the amount of anti-SARS-CoV-2
immunoglobulins (IgG, IgM) in the blood or plasma.
However, with this method, it is very quick and easy to determine if a person has been infected with the virus before and despite the positive features of this method, which is widely used today to diagnose and identify people with the corona virus, but in this method, the disease cannot be correctly diagnosed in the early stages of infection with the virus And it should be a few days after the person becomes infected with the virus [32,33]. Reverse transcription-polymerase chain reaction (RT-PCR) is a convenient clinical test in which sampling is performed from pharyngeal swab or an oral swab [3]. Swabs must be refrigerated to be safely transferred to a microbiology laboratory. RT-PCR is an accurate diagnostic method that diagnoses infection to SARS-CoV-2, at the acute stage of infection [11]. COVID-19 diagnosis kits target regions on a gene that codes for the protein that makes the virus’s nucleocapsid, an envelope that houses its RNA [34,35]. As the next stage for the certain diagnosis, computerized tomography (CT) imaging is used. So far, there were so many cases in which patients with negative result in RT-PCR test had CT test with positive result [31]. The main deficiency of CT imaging method is no specific diagnosis of the infection. In the other words, the type of the infection (COVID-19 or other types) is not truly identified by CT imaging, but the disease progress in the lungs is clearly recognized [36]. It has been observed that the CTs of the COVID-19 patients are similar to other diseases that cause pneumonia [37,38].
Applied Treatment Methods for COVID-19
So far, WHO has recommended several drugs for treatment of COVID-19. Initially, broad spectrum interfrons-anebulization antiviral drugs were used, but only remdesivir showed a good effect on the coronavirus [39,40]. Remdesivir, a nucleotide analog drug, has been approved by the FDA as the first antiviral drug to treat COVID-19. Indeed, upon entering the host cell, the drug is metabolized to the metabolite alanine and converted to nucleoside triphosphate (NTP). This compound, which is similar to ATP, disrupts RNA transcription and eliminates the SARS-CoV-2 virus during RNA transcription [41]. Clinical trials have shown that the effective dose of this drug is equal to 200mg on the first day and from the second to the tenth day equal to 100mg per day in the diet of patients, which has shown good results in their treatment [42]. Chloroquine is an anti-malarial and autoimmune drug and has been reported to be a broad-spectrum antiviral drug Which is widely used. Chloroquine is known to block virus infection by raising the endosomal pH required for virus / cell fusion as well as interfering with the glycosylation of SARS-CoV cell receptors [43]. A combination of remdesivir and chloroquine demonstrated effectiveness by inhibition of SARS-CoV-2 [44]. Favipiravir (T-705) is a WHO-approved antiviral agent that is available in pill form. By inhibiting the RNA transcription of the virus cell, it prevents the virus from replicating or causing lethal mutagenesis for the virus cell, without causing toxicity to other cells. During studies on Vero E6 cells (ATCC-1586), it had a beneficial effect in preventing SARSCoV-2 infection without any significant side effects [45,46]. Whereas other antiviral drugs have shown mediocre results. Such antiviral drugs and antibiotics have been combined with traditional Chinese medicines and have been evaluated in humans and mice [47,48]. As another treatment method, recently, in Shanghai (China) injection of the blood plasma of the improved patients exhibited good results [21,49]. When a person becomes infected with the SARS-CoV-2 virus, The body begins to produce antibodies to fight the virus factor, hence, in this therapy, a part of the blood of the Treated people (which has antibodies) is injected into people infected with the virus or Susceptible to infection. The results showed that after plasma injection the antibody titer in the blood of these people increased rapidly so that at the time of their blood sampling, no RNA of the SARS-CoV-2 virus was detected, and the disease had cured. However, what are the limitations and challenges of this treatment? These challenges include matching blood types and the absence of other pathogenic viruses [50]. Therefore, studies were performed on the transmission of antibodies alone as a primary and uncomplicated treatment for COVID-19. The results of studies conducted by Group Wang, Y et al. [39] on COVID-19 patients with different disease severity showed a different pattern of antibody response and viral shedding. By examining different tissues in patients ‘bodies, they were able to obtain SARS-CoV-2 specific antibodies (IgM, IgG) outside the patients’ Respiratory Tract [51]. In fact, the immune system produces these antibodies, preventing the virus from Binding to the cell surface and inhibiting the fusion of the host membrane. The results showed that this treatment method had a quick recovery in patients [50,52].
Recent studies have shown that a significant number of deaths in patients with COVID-19 are according to an overactive immune system. Hence, in order to relieve pain and inflammation, during studies performed on patients with different intensities of the disease, 6 mg of Dexamethasone was administered orally or intravenously for 10 days and showed a 35% reduction in death. Therefore, this drug was approved as one of the life-saving drugs for patients infected with COVID-19 virus [53]. Macrolides such as azithromycin or clarithromycin are a class of immunomodulatory and anti-inflammatory antibiotics that their coadministration with hydroxychloroquinone seemed to be good candidate for the treatment of COVID-19 [54,55]. In one of the recent reports, the role of chloroquine and hydroxychloroquine in viral load was investigated, by Gautret et al. [56] (from IHU-Méditerranée Infection, Marseille, France) [56]. In their study, during the treatment period (from first to 16th of March/2020), 600 mg of hydroxychloroquine was administrated to the patients in three servings, every day, and the viral load of the nasopharyngeal swabs were tested. Along that, a group of the infected people did not received hydroxychloroquine, as a control group. In this way, researchers found out that hydroxychloroquine is effective in clearing the nasopharyngeal space of the virus and even the addition of azithromycin accelerates the process of recovery of the patients. However, upon publishing the Gautret’s report, so many attentions were attracted to coadministration of macrolides with hydroxychloroquinone. A few days later, Gautret’s claim was critically discussed by Machiels et al. [57] (from Department of Medical Microbiology & Radboudumc Center for Infectious Diseases, the Netherlands) [57]. In Machiels’s article entitled “Reply to Gautret et al. [57] it was expressed that there is no adequate evidence for the effectiveness of coadministration of hydroxychloroquine and macrolides to the COVID-19 patients. Machiels et al. [57] declared there would be so many negative side effects to the mentioned medicinal compilation, and the patients will be exposed to high risk via this method. In this way, another report that was published by “The Lancet” journal in 22/May/2020 and included some analytical data about the effectiveness of coadministration of hydroxychloroquine and macrolides, was immediately withdrawn [58]. Given these issues, it is found out that the risk of using these drugs is high in particular for the patients with cardiovascular disease due to their relationship with electrical instability [58]. The efficacy of the combination of hydro chloroquine and azithromycin against COVID-19 has become a very controversial issue in the medical community.
Type 1 interferon (IFN-I) that plays an important role in antiviral immunity, can also be a safe and easy probable treatment for COVID-19, as well [59]. IFN-I is a large class of interferon made of protein and its main responsibility is to assist the regulation of the activity of the human immune system. IFN-I includes a group of cytokines including subgroups alpha and beta, which are themselves divided to other isoforms. Briefly, when pattern recognition receptors (PRRs) detect a viral ingredient in the blood serum, the IFN-I are immediately secreted. IFNAR receptors present at the cell membrane detect the secreted IFN-I through fixation by transcriptional factors. The IFN-I should be used in the early stages of the infection to prevent the side effects and optimize antiviral therapy [60,61]. Recently, it was administrated to the COVID-19 patients in combination with ribavirin [59-61]. As a big bug, it should seriously be noted that excessive use of the interferon causes damages to the healthy tissues and in some cases, anti-interferon drugs is necessary to be administrated to reduce the possible damages [62].
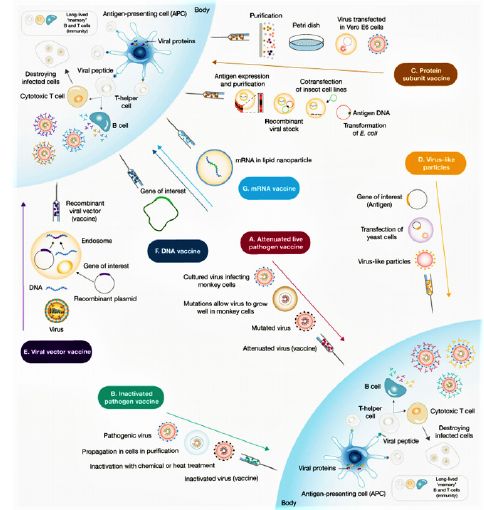
Vaccines typically contain agents similar to disease-causing microorganisms, including: live attenuated vaccine(they are made by genetically engineering the target virus to stimulate the immune system and produce antibodies without causing disease) [63,64], protein-based vaccines (contains supramolecular structures or recombinant proteins that are similar to viral proteins) that known as virus-like particles (VLPs) and they stimulate the immune system to produce antibodies and memory cells [65]. These VLPs according to the lack of virus genetic material are safer than live attenuated vaccines [66,67]. Vector vaccines can also be divided into two categories: non-Replication vectors and replication vectors. Non-replication vaccines produce the vaccine antigen when they enter the cell but are unable to reproduce, while replication vectors infect cells when they enter the cell and in addition to stimulating the immune system and producing specific antigens of pathogens, they are able to reproduce infectious viral vectors and then infects the target cells. Hence, DNA vaccines (to transmit pathogenic genes based on plasmid DNA) and RNA vaccines (which are reproducible based on their mRNA or RNA) used as a strategy for the expression of immunogenic viral proteins [68]. Also, today, due to the covid 19 epidemic method, a large number of vaccines have been produced from the category of mRNA vaccines that have been designed according to S protein. As shown in Figure 3 the main mechanism of these vaccines is that they trigger an immune response by activating B cells and as a result, when body exposed to a secondary antigen, antibodies produced in the plasma bind to the virus antigens and prevent the virus from binding to the target cell [69]. Remarkably, mRNA vaccines are not very stable, so for degradation, they are synthesized with modified nucleotides, such as lipid nanoparticles. One of the designed vaccines is the ChAdOx1 nCoV-19 vaccine, which it contains the Cemenia adenovirus vector with a defective ChAdOx1 replication and contains the complete spike protein SARS-CoV-2 and high level of neutralizing and induced antibodies without any serious side effects [70] (Figure 3).
In this predicament, pharmaceutical companies around the world have been trying to find a definitive and specific vaccine to treat the SARS-COV-2. After a year of research in this field, a number of these companies have succeeded in approving their vaccine in the World Health Organization that table 1 shows the first and most important vaccines. BNT162b2 is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 spike protein. After injection, this vaccine stimulates the body and triggers an immune response and the production of specific SARS-CoV-2 antibodies. In studies, 43,548 participants were randomly selected from people over 16 years of age and BNT162b2 was injected at 30 micrograms per dose in two doses 21 days apart. The results showed minor side effects such as mild to moderate pain at the injection site, headache, and fatigue, as well as protection and efficacy of over 95% of patients against Covid-19. Therefore, this vaccine was approved by WHO on 31/12/2020 according to its high efficacy and low side effects. But with all the benefits of this vaccine, there is a bug to how it is stored, which requires very low temperatures. Therefore, the research continued in such a way that in addition to using this vaccine, a vaccine would produce that could be easily stored and sent to other countries [72]. mRNA-1273 was another vaccine Was unveiled by Modrena Manufacturer in US that the advantage of this vaccine over the BNT162b2 is that it does not require very low temperatures to maintain it. The mRNA-1273 vaccine is a lipid nanoparticle– encapsulated mRNA-based vaccine that encodes the prefusion stabilized full-length spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In clinical trials performed on eligible patients over 18 years of age by intramuscular injection according to the diet, two doses were injected with an interval of 28 days, with a concentration of 0.2mg/ml [73]. High levels of binding and neutralizing antibodies have been observed during the studies. This vaccine also had shown 94.5% efficacy in a recent Phase 3 trial [74]. No serious side effects have been reported for this vaccine. The only side effects that were observed after 7 days of vaccination included mild side effects of weakness, headache, chills, myalgia and low fever, which were more after the second dose of the vaccine [75,76]. In March - April 2021 Ad26.COV2. S vaccine was approved by the WHO as a useful vaccine for the treatment of COVID 19. This vaccine is a recombinant vector of adenovirus serotype 26 (Ad26) that encodes a SARS-CoV-2 surface-stabilized spike protein. Therefore, when it enters the body, it stimulates CD4 + T-cell cells and produces IgG1 and IgG3 antibodies. Studies in participants aged 18 to 60 years have shown 85% efficiency with low side effects such as pain at the injection site, headache, abdominal pain, fatigue and nausea [77,78]. The ChAdOx1 nCoV-19 vaccine (AZD1222) that was developed at Oxford University, consists of a replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the SARSCoV- 2 structural surface glycoprotein antigen or spike protein gene. In a study of 23,848 participants aged 18 to 55 who were randomly selected from across Brazil, the United Kingdom and South Africa and the vaccine was given to them in one or two doses, after each injection of this vaccine, the immune system showed acceptable immune profile for the vaccine by inducing neutralizing antibodies and antigen-specific T cells against the SARS-CoV-2 spike protein. According to the results, protection was 64·1% after the first dose and 70·4% after second injection without any side effects or even death in AZD1222 receptors. This vaccine was approved by the WHO as a suitable vaccine for global distribution as a highperformance vaccine for the treatment of COVID 19 on 15 Feb 2021. But the remarkable thing about this vaccine is that it needs 2-8 degrees Celsius for storage, so it can be a challenge for low-income countries to use [79,80].
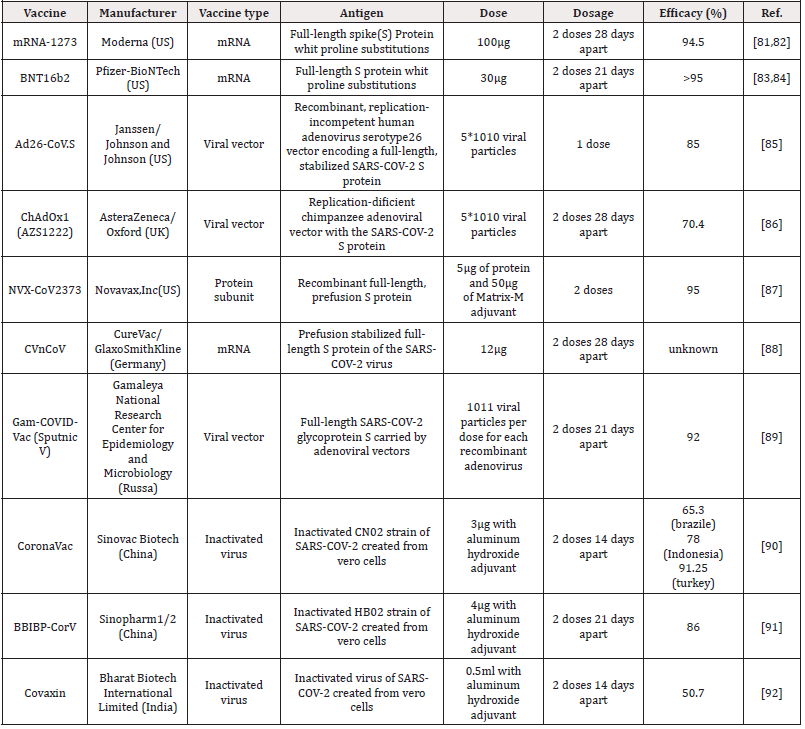
Table 1: Safe and effective vaccines against COVID-19 which had been registered with the World Health Organization.
Mutations
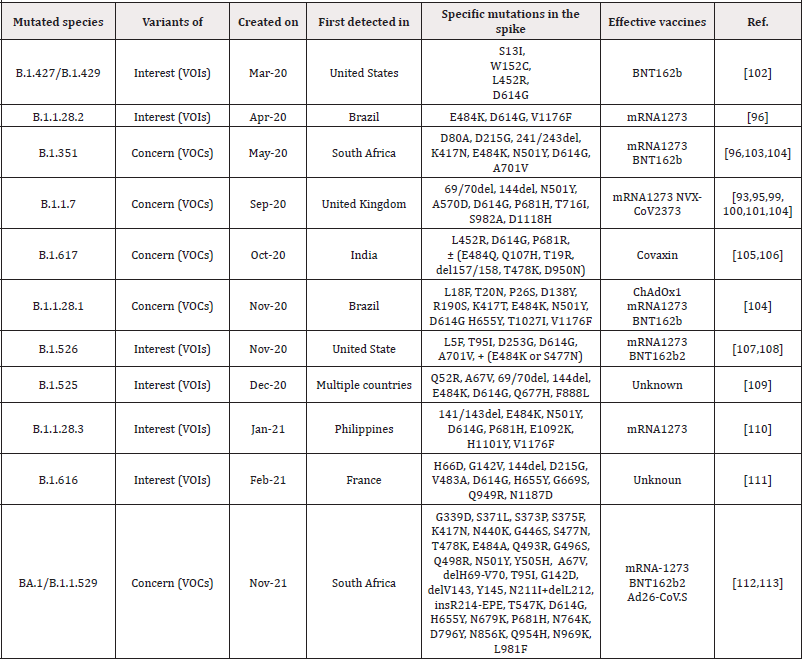
As COVID-19 progresses, there are opportunities for the SARS-CoV-2 virus to cause possible mutations in it, which help to evade from the immune system and spread the virus. One of the mutations in SARS-CoV-2 virus is a mutation in the virus spike protein, which is a special concern of researchers today, because these glycoproteins mediate the binding to ACE2 receptors and the entry of the virus into the target cell, as well as the main target of antibodies to neutralize them. As a result, mutations in it can lead to increased disease intensity, resistance to immunity and vaccines, and the onset of new disease symptoms [93,94]. Such mutations in the SARS-CoV-2 virus that have led to the global spread of those species, include type B.1.1.7(It was first identified in September 2020 in the UK) This species has 17 mutations in its original genome (SARS-COV-2), which has increased its efficiency for transmission to other humans. 8 of these 17 mutations has been created in spike protein of virus. Of these, 3 mutations including: N501Y (Mutation in the remainder of the main binding of viral spike to the target cell receptor, which resulted to increased desire for binding to the ACE2 receptor), P681H mutation at the site of the furin gap in the virus spike (This area is important for the transmission and infection of the disease) and ΔH69-V70 mutation are very important which increase the risk of virus infection and evade from the immune system [93,95]. Type B.1.1.248 (P.1) The Brazilian type that has been evolving since February 2020), It contains 11 mutations in its S proteins that the three most important of these mutations are N501Y, K417T and E484K, which increases the infection and accelerates the transmission from one person to another [96]. Type B.1.351(The type of South Africa that separated from the mouth and throat swabs of South African patients in November 2020) This type of mutation includes several mutations in its structural and non-structural proteins in compared with SARS-COV-2 virus. This species, like B.1.1.248, has three important mutations, N501Y, K417T and E484K, which increase the efficiency of virus binding to target cells and increase infection [96]. Type BA.1/B.1.1.529 is the latest mutation variant that was discovered in Botswana in November 2021 and reported to the World Health Organization (WHO) as a novel variant containing an unprecedented number of previously described and novel mutations. There are up to 59 mutations in this variant’s genome, with 36 of them in the spike protein. [97] As you know, in addition to these mutations, other mutations are B.1.617 (Indian species which itself divided into 3 subcategories B.1.617.1, B.1.617.2 and B.1.617.3 and have different types of mutations and phototypic characteristics, B.1.427 / B.1.429 (This species was created in the United States), B.1.616 (France) and .... The complete data set of these mutations has been brought in (Table 2). In fact, in all of this, mutations were created for Increased resistance to vaccines and the immune system and also increase transmission from person to person. But the very important challenge is, can these mutations, which are form in different species, be treated with the IgG and IgM antibodies or available vaccines? (Figure 4).
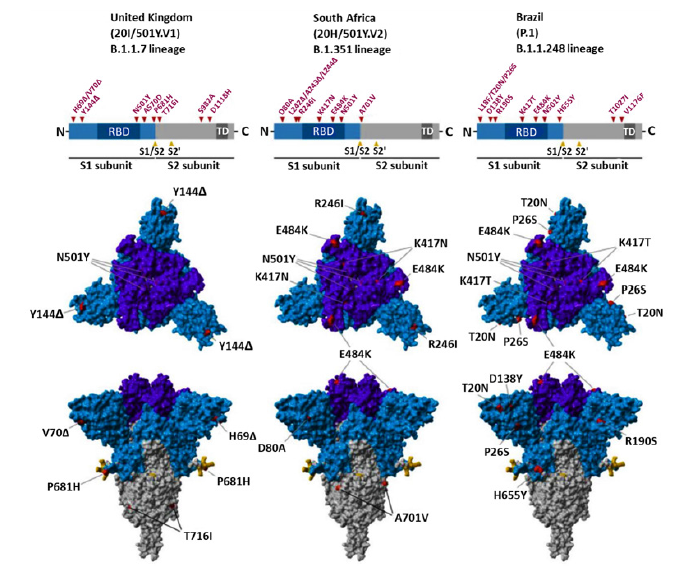
Figure 4:Genome, S-protein structure and location of mutations in UK, South Africa and Brazil variant [98].
Studies by Wu et al. On the neutralization rate of human and non-human serum infected with B.1.1.7 who were vaccinated with the mRNA-1273 vaccine demonstrated an effective neutralization response to this vaccine [99]. Also, during a survey that performed by Xiaoying Shen et al. On B.1.1.7-infected serums, they found that Moderna and Novavax vaccines were very effective in treating this mutant species (B.1.1.7) so that, the Novavax vaccine was 85.6% effective in treating B.1.1.7 [100]. According to a study by Markus Hoffmann Group, it was observed that monoclonal antibodies that were effectively used to treat COVID-19 do not completely prevent the guided entry by S proteins of B.1.1.248 and B.1.351 virus surface [98]. Furthermore, in another study by David Harrington et al. on patients with a history of SARS-CoV-2, Even before infection with B.1.1.7, they had anti-SARS-CoV-2 antibodies in their blood serum and there was no evidence of a decrease in serum antibodies [101]. As a result, it can be assumed that, depending on the situation and the type of mutation that occurs in SARS-CoV-2, it may or may not have serious effects on the new mutant types and considering that there are so many different possibilities in creating a mutation, a comprehensive interpretation cannot be given and in fact, a lot of research needs to be done to treat any new ones that are created. In order to deal with new mutation type, a very targeted and highly effective treatment method can be provided to eradicate the virus.
Suggested Therapeutic Strategy for COVID-19
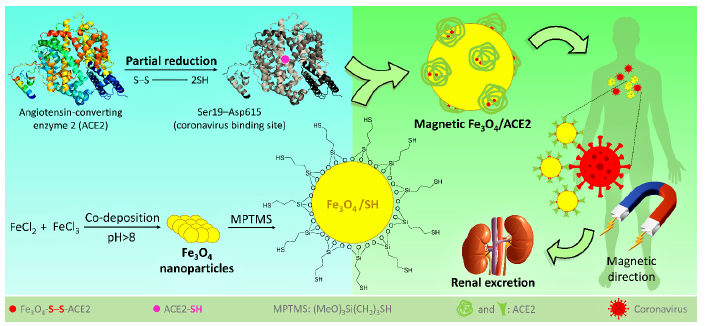
As a result, it seems that one effective trick that we can think of is to try to collect the SARS-CoV-2 by administration of the fake receptors (ACE2), to reduce the chance of the original ACE2 (on the lung’s cells) for the attachment. Then, this is essential to lead the collected ACE2/SARS-CoV-2 complex to immediate excretion. In the way of COVID-19 treatment, injection of the fake angiotensinconverting enzyme 2 (ACE2) (the receptor of the coronavirus) into the blood serum has been recently suggested by the researchers [114]. This protein is located onto the surface of many cell types (lungs, heart, blood vessels, kidneys, liver and gastrointestinal tract), and the exchange of O2 and CO2 between the lungs and blood vessels is its main function [115,116]. In COVID-19, ACE2 provides the entry point for the coronavirus to attach to the lung’s cells and as a result the normal function of the ACE2 is inhibited [116]. In fact, the coronavirus crosses a key-lock model with the ACE2 receptor, which is well-known in antibody-antigen attachments, to hook into and infect the lung’s cells [114-117]. Accordingly, it is quite possible and reasonable to use the fake ACE2 receptors to capture the coronavirus and lead them to the kidneys and ultimately renal excretion. For this purpose, we only need to practically preserve the native structure of the ACE2 by working at very low temperatures. Like other protein species, at high temperatures or intense conditions, the ACE2 is denatured, and its reception function is lost. From the structural aspect, ACE2 includes zinc ion (Zn2+) and also so many functional groups containing the oxygen and nitrogen atoms, coming from the side chains of different amino acids [118]. According to a recent docking study published in the current year (2020) by Nature publications [119], it was revealed that the N-terminal peptidase domain (Ser19-Asp615) and the zinc ion of ACE2 are involved in the attachment of receptor-binding domain of coronavirus. According to the literature, there is a disulfide (S-S) bond at subdomain I of ACE2 [118], which can be reduced to thiol sites via a partial reduction by mild reagents such as dithiothreitol (DTT), tris(2-carboxyethyl) phosphine (TCEP), and 2-mercaptoethanol [120]. Then, the resulted thiol sites in ACE2 could be used for the formation of a stable covalent bond to another thiol group (during an oxidation process).
Previously, we have reported so many achievements on the magnetic nanoparticles (especially iron oxide - Fe2O3 and Fe3O4), which are functionalizable due to including chemical functional groups onto the surfaces [121-125]. We have widely used Fe3O4 nanoscale magnetic core for various applications such as organic catalysis [126-129], drug carrier [130], and photocatalysis for degradation of the hazardous substances [131,132]. From the structural aspect, Fe3O4 nanoparticles include hydroxyl groups (-OH) onto their surfaces that are considered as an appropriate site for making a covalent bond with chemical/biochemical structures. Moreover, there are several additional advantages for using this type of materials such as low cost and convenient preparation, high magnetic property, nontoxicity, biodegradability, tiny size, convenient separation, etc. In our previous report, we have demonstrated that the surface of Fe3O4 nanoparticles could be easily functionalized with thiol-containing organic structures via different strategies [133]. Moreover, its great magnetic behavior gives us this substantial opportunity to direct the particles to the target tissue in the body’s internal environment, just by applying an external magnetic field [134].
Herein, we intend to suggest an applicable efficient approach to capture the coronavirus before attachment to the original ACE2, and direct that to the kidneys for renal excretion. Since the treatment process of the COVID-19 disease deeply depends on the strength of the body’s immune system (which is different for everybody), it obtains so much importance to shorten the treatment time, especially for someone who does not have so strong immune system. For this purpose, it is suggested to prepare ACE2-functionalized magnetic nanoparticles with the size of <50nm diameter. Then, the particles are magnetically directed to the kidneys, which is a relatively fast process than the normal flow of the blood serum circulation in the body. In this way, the fake ACE2 is tagged onto the surface of the Fe3O4 nanoparticles via stable covalent binding and subjected to the infected blood serum. This is so important to preserve the native structure of the ACE2 during the attachment onto the surfaces. Otherwise, the virus-connection specific function of the fake ACE2 will be lost. One of the most crucial conditions for working on the native proteins is to reduce the temperature to below 4℃ [135]. Also, this is an important issue to prepare tiny scale particles (smaller than 20 nm) due to having a successful renal excretion [136]. (Figure 5), schematically presents the suggested preparation route of Fe3O4/ACE2 magnetic receptor for coronavirus and further therapeutic method. (Figure 5).
Conclusion
In late 2019, a novel viral infection emerged in Wuhan (China), through which a lot of people around the world lost their lives and a huge variation was occurred in the world’s population growth trend. Immediately, researchers and specialists started to work on the origin of the mentioned infection, which is called “SARS-CoV-2”. Everyone knows that the discovery of a vaccine for SARS-CoV-2 (as the main prophylaxis) and production will take a long time. During this time, finding the best treatment for the COVID-19 patients is essential to reducing mortality. In this way, some medications such as lopinavir, ritonavir, umifenovir and chloroquine were approved by WHO and administrated to the patients, to prevent the disease progress. As other types of the viruses, SARS-CoV-2 is entered into the target cells (lung) via attachment to its specific receptor. Therefore, one of the most logical methods that comes to the mind is to inject fake receptors to the patient’s blood serum to prevent attachment of the virus to the original receptors (ACE2, in the lung’s cells). Herein, we suggest a novel approach that is a compilation of nano- and biotechnology and seems to be applicable as a suitable treatment for COVID-19.
Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology (IUST).
Conflict of Interest
The authors declare no conflict of interest.
References
- Ping Ing Lee, Po Ren Hsueh (2020) Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect 53(3): 365-367.
- Qifang Bi, Yongsheng Wu, Shujiang Mei, Chenfei Ye, Xuan Zou, et al. (2020) Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 20(8) 911-919.
- Fei Zhou, Ting Yu, Ronghui Du, Guohui Fan, Ying Liu, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet 395: 1054-1062.
- Tao Zhang, Qunfu Wu, Zhigang Zhang (2020) Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol 30(7): 1346-1351.
- Aisha M Al Osail, Marwan J Al Wazzah (2017) The history and epidemiology of Middle East respiratory syndrome corona virus. Multidiscip Respir Med 12: 20.
- Pavan Kumar Samudrala, Pramod Kumar, Kamlesh Choudhary, Nagender Thakur, Gaurav Suresh Wadekar et al. (2020) Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur J Pharmacol 883: 173375.
- Nas FS, Ali M, Muazu L, Salihu Abdallah M (2020) Epidemiology, virology, pathogenesis and treatment of novel COVID-19. J Cur Tre Pharm Sci 1 e103.
- Yuefei Jin, Haiyan Yang, Wangquan Ji, Weidong Wu, Shuaiyin Chen (2020) Virology, Epidemiology, pathogenesis, and control of COVID-19. Viruses 12(4): 372.
- Li Runfeng, Hou Yunlong, Huang Jicheng, Pan Weiqi, Ma Qinhai, et al. (2020) Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res156: e104761.
- Peng Zhou, Xing Lou Yang, Xian Guang Wang, Ben Hu, Lei Zhang, et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270-273.
- Sarah Ee Fang Yong, Danielle Elizabeth Anderson, Wycliffe E Wei, Junxiong Pang, Wan Ni Chia, et al. (2020) Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect Dis 20(7): 809-815.
- Hisashi Kai, Mamiko Kai (2020) Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res 43: 648-654.
- Junwen Luan, Yue Lu, Xiaolu Jin, Leiliang Zhang (2020) Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem. Biophys. Res. Commun 526(1): 165-169.
- Wendong Li, Zhengli Shi, Meng Yu, Wuze Ren, Craig Smith, et al. (2005) Bats are natural reservoirs of SARS-Like coronaviruses. Science 310(5748): 676-679.
- Susanna K P Lau, Hayes K H Luk, Antonio C P Wong, Kenneth S M Li, Longchao Zhu, et al. (2020) Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis 26(7): 1542-1547.
- Roujian Lu, Xiang Zhao, Juan Li, Peihua Niu, Bo Yang, et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 395: 565-574.
- Abel P David, Nicole T Jiam, Joshua M Reither, Jose G Gurrola, Manish K Aghi et al. (2020) Endoscopic skull base and transoral surgery during COVID‐19 pandemic: Minimizing droplet spread with negative‐pressure otolaryngology viral isolation drape. Head Neck-J Sci Spec 42: 1577-1582.
- Roujian Lu, Xiang Zhao, Juan Li, Peihua Niu, Bo Yang et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 395 (10224): 565-574.
- Christine V F Carrington, Jerome E Foster, Hua Chen Zhu, Jin Xia Zhang, Gavin J D Smith, et al. (2008) Detection and phylogenetic analysis of group 1 coronaviruses in South American bats. Emerg. Infect. Dis 14(12): 1890-1893.
- Dua D, Yadav M, Jetley P, Dua R (2020) Covid-19: Immunological lessons from bats, pangolins and old coronaviruses; and how we can apply them in a timely way for a better outcome. Preprints 2020040071.
- Muhammad Adnan Shereen, Suliman Khan, Abeer Kazmi, Nadia Bashir, Rabeea Siddique, et al. (2020) COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res 24: 91-98.
- Barik S (2020) Genus-specific pattern of intrinsically disordered central regions in the nucleocapsid protein of coronaviruses. Comput Struct. Biotec 18: 1884-1890.
- Aiping Wu, Yousong Peng, Baoying Huang, Xiao Ding, Xianyue Wang, et al. (2020) Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27(3): 325-328.
- Alexandra C Walls, Young Jun Park, M Alejandra Tortorici, Abigail Wall, Andrew T McGuire Veesler, et al. (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181(2): 281-292.
- Markus Hoffmann, Hannah Kleine Weber, Simon Schroeder, Nadine Krüger, Tanja Herrler, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2): 271-280.
- Cynthia Liu, Qiongqiong Zhou, Yingzhu Li, Linda V Garner, Steve P Watkins, et al. (2020) Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases, ACS central sci 315-331.
- Jian Shang, Yushun Wan, Chang Liu, Boyd Yount, Kendra Gully et al. (2020) Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog 16(3): e1008392.
- Qiao Shi, Xiaoyi Zhang, Fang Jiang, Xuanzhe Zhang, Ning Hu et al. (2020) Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care 43(7): 1382-1391.
- Weina Guo, Mingyue Li, Yalan Dong, Haifeng Zhou, Zili Zhang, et al. (2020) Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes-Metab. Res 36(7): e3319.
- Moneriz C (2020) Fármacos prometedores y potenciales para el tratamiento de COVID-19. Revista chilena de infectología 37(3): 205-215.
- François Bénézit, Paul Le Turnier, Charles Declerck, Cécile Paillé, Matthieu Revest, et al. (2020) Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis 20(9):1014-1015.
- Corine H Geurtsvan Kessel, Nisreen M A Okba, Zsofia Igloi, Susanne Bogers, Carmen W E Embregts, et al. (2020) An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. commun 11(1): 1-5.
- Mayara Lisboa Bastos, Gamuchirai Tavaziva, Syed Kunal Abidi, Jonathon R Campbell, Louis Patrick Haraoui et al. (2020) Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. Bmj 370: m2516.
- Kumar Vashist S (2020) In Vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagnostics 10(4): e202.
- Jianguo Wu, Jiasheng Liu, Shijun Li, Zhiyang Peng, Zhe Xiao, et al. (2020) Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Di 37:101673.
- Heshui Shi, Xiaoyu Han, Nanchuan Jiang, Yukun Cao, Osamah Alwalid, et al. (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis 20(4): 425-434.
- Di Dong, Zhenchao Tang, Shuo Wang, Hui Hui, Lixin Gong, et al. (2020) The role of imaging in the detection and management of COVID-19: a review. IEEE T Bio-Med. Eng 14:16-29.
- Harrison X Bai, Ben Hsieh, Zeng Xiong, Kasey Halsey, Ji Whae Choi, et al. (2020) Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology 296(2): E46-E54.
- Yeming Wang, Dingyu Zhang, Guanhua Du, Ronghui Du, Jianping Zhao, et al. (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet 395(10236): 1569-1578.
- Jonathan Grein, Norio Ohmagari, Daniel Shin, George Diaz, Erika Asperges, et al. (2020) Compassionate use of remdesivir for patients with severe Covid-19. N Engl J 382(24): 2327-2336.
- M Gabriella Santoro, Ernesto Carafoli, et al. (2021) Remdesivir: from Ebola to COVID-19. Biochem Biophys Res Commun 538: 145-150.
- Sarah C J Jorgensen, Razieh Kebriaei, Linda D Dresser (2020) Remdesivir: review of pharmacology, pre‐clinical data, and emerging clinical experience for COVID‐19. Pharmacology 40(7): 659-671.
- Franck Touret, Xavier de Lamballerie (2020) Of chloroquine and COVID-19. Antiviral res 177: 104762.
- Liying Dong, Shasha Hu, Jianjun Gao (2020) Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther14(1): 58-60.
- Zarir F Udwadia, Pawan Singh, Hanmant Barkate, Saiprasad Patil, Shabbir Rangwala, et al. (2021) Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis 103: 62-71.
- Morteza Ghasemnejad Berenji, Sarvin Pashapour (2020) Favipiravir and COVID-19: a simplified summary. Drug Res 71(03): 166-170.
- Jaffar A Al Tawfiq, Ali H Al Homoud, Ziad A Memish (2020) Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis 34: e101615.
- Ka Tim Choy, Alvina Yin Lam Wong, Prathanporn Kaewpreedee, Sin Fun Sia, Dongdong Chen, et al. (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res 178: e104786.
- Anne Catherine Cunningham, Hui Poh Goh, David Koh (2020) Treatment of COVID-19: old tricks for new challenges. Critical Care 24(1): 91.
- Abraham J (2020) Passive antibody therapy in COVID-19. Nat Rev Immunol 20(7): 401-403.
- Yanqun Wang, Lu Zhang, Ling Sang, Feng Ye, Shicong Ruan et al. (2020) Kinetics of viral load and antibody response in relation to COVID-19 severity. J clin Invest 130(10): 5235-5244.
- Sabra L Klein, Andrew Pekosz, Han Sol Park, Rebecca L Ursin, Janna R Shapiro, et al. (2020) Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J clin Invest 130(11): 6141-6150.
- Twan Lammers, Alexandros Marios Sofias, Roy van der Meel, Raymond Schiffelers, Gert Storm, et al. (2020) Dexamethasone nanomedicines for COVID-19. Nat nanotechnol 15(8): 622-624.
- Julien Andreani, Marion Le Bideau, Isabelle Duflot, Priscilla Jardot, Clara Rolland et al. (2020) In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog 145: 104228.
- Matthieu Million, Jean Christophe Lagier, Philippe Gautret, Philippe Colson, Pierre Edouard Fournier, et al. (2020) Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis 35: 101738.
- Gautret, P (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56(1): 105949.
- Julian D Machiels, Chantal P Bleeker Rovers, Rob Ter Heine, Janette Rahamat Langendoen, Quirijn de Mast, et al. (2020) Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID-19: what is the evidence and what are the risks?. Int J Antimicrob Agents 56(1): 106056.
- Mandeep R Mehra, Sapan S Desai, Frank Ruschitzka, Amit N Patel, et al. (2020) RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet S0140-6736(20): 31180-31186.
- Erwan Sallard, François Xavier Lescure, Yazdan Yazdanpanah, France Mentre, Nathan Peiffer Smadja (2020) Type 1 interferons as a potential treatment against COVID-19. Antivir Res 178: 104791.
- Acharya D, Liu GQ, Gack MU (2020) Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol 20(7): 397-398.
- Park A, Iwasaki A (2020) Type I and type III interferons-induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27(6): 870-878.
- Dritschilo A, Mossman K, Gray M, Sreevalsan T (1982) Potentiation of radiation injury by interferon. Am J Clin Oncol 5(1): 79-82.
- Plotkin SA (2009) Vaccines: the fourth century. Clin Vaccine Immunol 16(12): 1709-1719.
- Ehrenfeld E, John Modlin, Konstantin Chumakov (2009) Future of polio vaccines. Expert Rev Vaccines 8(7): 899-905.
- Zeltins A (2013) Construction and characterization of virus-like particles: a review. Mol Biotechnol 53(1): 92-107.
- Lu J, Guoliang Lu, Shudan Tan, Jia Xia, Hualong Xiong, et al. (2020) A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res 30(10): 936-939.
- Crisci E, Juan Bárcena, María Montoya (2012) Virus-like particles: the new frontier of vaccines for animal viral infections. Vet Immunol Immunopathol 148(3-4): 211-225.
- Vogel AB, Laura Lambert, Ekaterina Kinnear, David Busse, Stephanie Erbar, et al. (2018) Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther 26(2): 446-455.
- Park JW, Philip NP Lagniton, Yu Liu, Ren He Xu (2021) mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci 17(6): 1446-1460.
- Haibo Zhang, Josef M Penninger, Yimin Li, Nanshan Zhong, Arthur S Slutsky (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4): 586-590.
- Nikolaos C Kyriakidis, Andrés López Cortés, Eduardo Vásconez González, Alejandra Barreto Grimaldos, Esteban Ortiz Prado (2021) SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 6(1): 28.
- Fernando P Polack, Stephen J Thomas, Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, et al. (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med 383(27): 2603-2615.
- Lindsey R Baden, Hana M El Sahly, Brandon Essink, Karen Kotloff, Sharon Frey, et al. (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med 384(5): 403-416.
- Alicia T Widge, Nadine G Rouphael, Lisa A Jackson, Evan J Anderson, Paul C Roberts, et al. (2021) Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med 384(1): 80-82.
- Kai Wu, Anne P Werner, Juan I Moliva, Matthew Koch, Angela Choi, et al. (2021) mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. BioRxiv 2021.01.25.427948.
- Srikrishna V Malayala, Gisha Mohan, Deepa Vasireddy, Paavani Atluri (2021) Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus 13(3): e14099.
- Jerald Sadoff, Glenda Gray, An Vandebosch, Vicky Cárdenas, Georgi Shukarev, et al. (2021) Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med 384(23): 2187-2201.
- Jerald Sadoff, Mathieu Le Gars, Georgi Shukarev, Dirk Heerwegh, Carla Truyers, et al. (2021) Interim Results of a Phase 1-2a Trial of Ad26. COV2. S Covid-19 Vaccine. N. Engl. J. Med 384(19): 1824-1835.
- Merryn Voysey, Sue Ann Costa Clemens, Shabir A Madhi, Lily Y Weckx, Pedro M Folegatti, et al. (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397(10269): 99-111.
- Katie J Ewer, Jordan R Barrett, Sandra Belij Rammerstorfer , Hannah Sharpe, Rebecca Makinson, et al. (2021) T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nature Med 27(2): 270-278.
- Lisa A Jackson, Evan J Anderson, Nadine G Rouphael, Paul C Roberts, Mamodikoe Makhene, et al. (2020) An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med 383(20):1920-1931.
- Evan J Anderson, Nadine G Rouphael, Alicia T Widge, Lisa A Jackson, Paul C Roberts, et al. (2020) Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults N Engl J Med 383(25): 2427-2438.
- Fernando P Polack, Stephen J Thomas, Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, et al. (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(27): 2603-2615.
- Matan Levine Tiefenbrun, Idan Yelin, Rachel Katz, Esma Herzel, Ziv Golan et al. (2021) Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nature Med 27(5):790-792.
- Jerald Sadoff, Glenda Gray, An Vandebosch, Vicky Cárdenas, Georgi Shukarev, et al. (2021) Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med 384(23): 2187-2201.
- Katherine R W Emary, Tanya Golubchik, Parvinder K Aley, Cristina V Ariani, Brian Angus, et al. (2021) Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B. 1.1. 7): an exploratory analysis of a randomised controlled trial. The Lancet 397(10282): 1351-1362.
- Jing Hui Tian, Nita Patel, Robert Haupt, Haixia Zhou, Stuart Weston, et al. (2021) SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat commun 12(1):372.
- Susanne Rauch, Nicole Roth, Kim Schwendt, Mariola Fotin Mleczek, Stefan O Mueller, et al. (2021) mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. Npj Vaccine 6(1): 1-9.
- Ian Jones, Polly Roy (2021) Sputnik V COVID-19 vaccine candidate appears safe and effective. The Lancet 397(10275): 642-643.
- Binay U (2021) Level of SARS-CoV-2 IgG antibodies after two doses CoronaVac vaccine. Primarily report.
- Shengli Xia, Yuntao Zhang, Yanxia Wang, Hui Wang, Yunkai Yang, et al. (2021) Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. The Lancet Infect. Dis 21(1): 39-51.
- Gajanan Sapkal, Pragya D Yadav, Raches Ella, Priya Abraham, Deepak Y Patil, et al. (2021) Neutralization of B. 1.1. 28 P2 variant with sera of natural SARS-CoV-2 infection and recipients of inactivated COVID-19 vaccine Covaxin. J Travel Med 28(7): taab077.
- Xiaoying Shen, Haili Tang, Charlene McDanal, Kshitij Wagh, William Fischer, et al. (2021) SARS-CoV-2 variant B. 1.1. 7 is susceptible to neutralizing antibodies elicited by ancestral Spike vaccines. Cell Host Microbe 29(4): 529-539.
- Venkata Viswanadh Edara, Carson Norwood, Katharine Floyd, Lilin Lai, Meredith E Davis Gardner, et al. (2021) Infection-and vaccine-induced antibody binding and neutralization of the B. 1.351 SARS-CoV-2 variant. Cell Host Microbe 29(4): 516-521.
- Nicholas G Davies, Sam Abbott, Rosanna C Barnard, Christopher I Jarvis, Adam J Kucharski et al. (2021) Estimated transmissibility and impact of SARS-CoV-2 lineage B. 1.1. 7 in England. Sci 372: (6538).
- Venkata Viswanadh Edara, Carson Norwood, Katharine Floyd, Lilin Lai, Meredith E Davis Gardner et al. (2021) Infection-and vaccine-induced antibody binding and neutralization of the B. 1.351 SARS-CoV-2 variant. Cell Host Microbe 29(4): 516-521.
- Xuemei He, Weiqi Hong, Xiangyu Pan, Guangwen Lu, Xiawei Wei, et al. (2021) SARS‐CoV‐2 Omicron variant: characteristics and prevention. MedComm 2(4):838-845.
- Markus Hoffmann, Prerna Arora, Rüdiger Groß, Alina Seidel, Bojan Hörnich, et al. (2021) SARS-CoV-2 variants B. 1.351 and B. 1.1. 248: Escape from therapeutic antibodies and antibodies induced by infection and vaccination. BioRxiv.
- Kai Wu Anne P Werner, Juan I Moliva, Matthew Koch, Angela Choi (2021) mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. BioRxiv 2021.01.25.427948.
- Xiaoying Shen, Haili Tang, Charlene McDanal, Kshitij Wagh, William Fischer, et al. (2021) SARS-CoV-2 variant B. 1.1. 7 is susceptible to neutralizing antibodies elicited by ancestral Spike vaccines. Cell Host Microbe 29(4): 529-539.
- David Harrington, Beatrix Kele, Spiro Pereira, Xose Couto Parada, Anna Riddell, et al. (2021) Confirmed Reinfection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variant VOC-202012/01. Clin. Infect. Dis 73(10):1946-1947.
- Karen B Jacobson, Benjamin A Pinsky, Maria E Montez Rath, Hannah Wang, Jacob A Miller, et al. (2021) Post-vaccination SARS-CoV-2 infections and incidence of the B. 1.427/B. 1.429 variant among healthcare personnel at a northern California academic medical center. medRxiv 2021.04.14.21255431.
- Daming Zhou, Wanwisa Dejnirattisai, Piyada Supasa , Chang Liu, Alexander J Mentzer et al. (2021) Evidence of escape of SARS-CoV-2 variant B. 1.351 from natural and vaccine-induced sera. Cell 184(9): 2348-2361.
- Jasdeep Singh, Jasmine Samal, Vipul Kumar, Jyoti Sharma, Usha Agrawal, et al. (2021) Structure-Function Analyses of New SARS-CoV-2 Variants B. 1.1. 7, B. 1.351 and B. 1.1. 28.1: Clinical, Diagnostic, Therapeutic and Public Health Implications. Viruses 13(3): 439.
- Pragya D Yadav, Gajanan N Sapkal, Priya Abraham, Raches Ella, Gururaj Deshpande, et al. (2021) Neutralization of variant under investigation B. 1.617 with sera of BBV152 vaccinees. bioRxiv.
- Pragya D Yadav, Gajanan N Sapkal, Priya Abraham, Gururaj Deshpande, Dimpal A Nyayanit, et al. (2021) Neutralization potential of Covishield vaccinated individuals sera against B. 1.617. 1. Clin Infect Dis 74(3): 558-559.
- Thompson C N (2021) Rapid Emergence and Epidemiologic Characteristics of the SARS-CoV-2 B. 1.526 Variant—New York City, New York, Morbidity and Mortality Weekly Report 70(19): 712.
- Hao Zhou, Belinda M Dcosta, Marie I Samanovic, Mark J Mulligan, Nathaniel R Landau, et al. (2021) B. 1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. bioRxiv 2021.03.24.436620.
- Felicidade Pereira, Stephane Tosta, Maricélia M Lima, Luciana Reboredo de Oliveira da Silva, Vanessa B Nardy, et al. (2021) Genomic surveillance activities unveil the introduction of the SARS‐CoV‐2 B. 1.525 variant of interest in Brazil: Case Report. J Med Virol 93(9):5523-5526.
- Francisco Barona Gómez, Luis Delaye, Erik Díaz Valenzuela, Fabien Plisson, Arely Cruz Pérez et al. (2021) Phylogenomics and population genomics of SARS-CoV-2 in Mexico reveals variants of interest (VOI) and a mutation in the Nucleocapsid protein associated with symptomatic versus asymptomatic carriers. medRxiv 7(11): 000684.
- World Health Organization (2021) COVID-19 Weekly Epidemiological Update.
- Shirley Collie, Jared Champion, Harry Moultrie, Linda Gail Bekker, Glenda Gray (2021) Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N England J M 386(5): 494-496.
- Wilfredo F Garcia Beltran, Kerri J St Denis, Angelique Hoelzemer, Evan C Lam, Adam D Nitido, et al. (2021) mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185(3): 457-466.
- Vanessa Monteil, Hyesoo Kwon, Patricia Prado, Astrid Hagelkrüys, Reiner A Wimmer et al. (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181(4): 905-913.
- Jian Zhong, Zhengchen Yan, Daoyan Liu, Yinxing Ni, Zhigang Zhao, et al. (2006) Association of angiotensin-converting enzyme 2 gene A/G polymorphism and elevated blood pressure in Chinese patients with metabolic syndrome. J Lab Clin Med 147(2): 91-95.
- Yuxuan Hou, Cheng Peng, Meng Yu, Yan Li, Zhenggang Han, et al. (2010) Angiotensin-converting enzyme 2 (ACE2) proteins of different bat species confer variable susceptibility to SARS-CoV entry. Arch Virol 155: 1563-1569.
- Hao Cheng, Yan Wang, Gui Qiang Wang (2020) Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. J Med Virol 92(7): 726-730.
- Paul Towler, Bart Staker, Sridhar G Prasad, Saurabh Menon, Jin Tang, et al. (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279(17): 17996-18007.
- Jun Lan, Jiwan Ge, Jinfang Yu, Sisi Shan, Huan Zhou et al. (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581(7807): 215-220.
- Singh R, Whitesides GM (1994) Reagents for rapid reduction of native disulfide bonds in proteins. Bioorg. Chem 22(1): 109-115.
- Maleki A, Taheri Ledari R, Soroushnejad M (2018) Surface functionalization of magnetic nanoparticles via palladium‐catalyzed Diels‐Alder approach. ChemistrySelect 3(46): 13057-13062.
- Taheri Ledari R, Rahimi J, Maleki A (2020) Method screening for conjugation of the small molecules onto the vinyl-coated Fe3O4/silica nanoparticles: highlighting the efficiency of ultrasonication. Mater Res Express 7: 015067.
- Ali Maleki, Reza Taheri Ledari, Jamal Rahimi, Mahya Soroushnejad, Zoleikha Hajizadeh (2019) Facile peptide bond formation: Effective interplay between isothiazolone rings and silanol groups at silver/iron oxide nanocomposite surfaces. ACS Omega 4(6): 10629-10639.
- Taheri Ledari R, Maleki A (2020) Antimicrobial therapeutic enhancement of levofloxacin via conjugation to a cell‐penetrating peptide: An efficient sonochemical catalytic process. J Pept Sci 26(10): e3277.
- Taheri Ledari R, Hashemi S M, Maleki A (2019) High-performance sono/nano-catalytic system: CTSN/Fe3O4-Cu nanocomposite, a promising heterogeneous catalyst for the synthesis of N-arylimidazoles. RSC Adv 9: 40348-40356.
- Jamal Rahimi, Reza Taheri Ledari, Ali Maleki (2019) Cellulose-supported sulfonated magnetic nanoparticles: utilized for one-pot synthesis of α-iminonitrile derivatives. Curr Org Synth 17(4): 288-294.
- Ali Maleki, Reza Taheri Ledari, Reza Ghalavand (2020) Design and fabrication of a magnetite-based polymer-supported hybrid nanocomposite: A promising heterogeneous catalytic system utilized in known palladium-assisted coupling reactions. Comb Chem High Throughput Screen 23(2): 119-125.
- Taheri Ledari R, Rahimi J, Maleki A (2019) Synergistic catalytic effect between ultrasound waves and pyrimidine-2,4-diamine-functionalized magnetic nanoparticles: Applied for synthesis of 1,4-dihydropyridine pharmaceutical derivatives. Ultrason. Sonochem 59: 104737.
- Maleki A, Taheri Ledari R, Ghalavand R, Firouzi Haji R (2020) Palladium-decorated o-phenylenediamine-functionalized Fe3O4/SiO2 magnetic nanoparticles: A promising solid-state catalytic system used for Suzuki-Miyaura coupling reactions. J Phys Chem Solids 136: 109200.
- Wenjie Zhang, Reza Taheri Ledari, Zoleikha Hajizadeh, Ehsan Zolfaghari, Mohammad Reza et al. (2020) Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide. Nanoscale 12: 3855-3870.
- Taheri Ledari R, Valadi K, Gharibi S, Maleki A (2020) Synergistic photocatalytic effect between green LED light and Fe3O4/ZnO-modified natural pumice: A novel cleaner product for degradation of methylene blue. Mater. Res. Bull 130: 110946.
- Hajizadeh Z, Valadi K, Taheri Ledari R, Maleki A (2020) Convenient Cr(VI) removal from aqueous samples: Executed by a promising clay‐based catalytic system, magnetized by Fe3O4 nanoparticles and functionalized with humic acid. ChemistrySelect 5(8) 2441-2448.
- Reza Taheri Ledari, Ali Maleki, Ehsan Zolfaghari, Maral Radmanesh, Hodjattallah Rabbani, et al. (2020) High-performance sono/nano-catalytic system: Fe3O4@Pd/CaCO3-DTT core/shell nanostructures, a suitable alternative for traditional reducing agents for antibodies. Ultrason Sonochem 61: 104824.
- Reza Taheri Ledari, Wenjie Zhang, Maral Radmanesh, Seyedeh Shadi Mirmohammadi, et al. (2020) Multi-stimuli nanocomposite therapeutic: docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small 16(41): 2002733.
- Alessandra Giugliarelli, Marco Paolantoni, Assunta Morresi, Paola Sassi (2012) Denaturation and preservation of globular proteins: The role of DMSO J Phys Chem B 116(45): 13361-13367.
- Hak Soo Choi, Wenhao Liu, Preeti Misra, Eiichi Tanaka, John P Zimmer et al. (2007) Renal clearance of nanoparticles. Nat. Biotechnol 25(10): 1165-1170.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.